if 50 ml of .114 m hno3 was added to 40 ml of the previous solution, what is the ph
Chapter 14. Acrid-Base Equilibria
14.7 Acid-Base Titrations
Learning Objectives
By the end of this section, you volition be able to:
- Interpret titration curves for potent and weak acid-base systems
- Compute sample pH at important stages of a titration
- Explain the part of acid-base of operations indicators
As seen in the affiliate on the stoichiometry of chemic reactions, titrations can be used to quantitatively analyze solutions for their acid or base concentrations. In this section, we will explore the changes in the concentrations of the acidic and basic species present in a solution during the procedure of a titration.
Titration Curve
Previously, when we studied acid-base reactions in solution, we focused but on the point at which the acid and base were stoichiometrically equivalent. No consideration was given to the pH of the solution before, during, or afterward the neutralization.
Example 1
Calculating pH for Titration Solutions: Strong Acrid/Stiff Base
A titration is carried out for 25.00 mL of 0.100 M HCl (strong acid) with 0.100 Grand of a strong base NaOH the titration curve is shown in Figure 1. Calculate the pH at these volumes of added base solution:
(a) 0.00 mL
(b) 12.50 mL
(c) 25.00 mL
(d) 37.50 mL
Solution
Since HCl is a strong acid, nosotros can assume that all of it dissociates. The initial concentration of H3O+ is [latex][\text{H}_3\text{O}^{+}]_0 = 0.100\;M[/latex]. When the base solution is added, it as well dissociates completely, providing OH− ions. The H3O+ and OH− ions neutralize each other, and then only those of the two that were in excess remain, and their concentration determines the pH. Thus, the solution is initially acidic (pH < 7), but eventually all the hydronium ions nowadays from the original acrid are neutralized, and the solution becomes neutral. Equally more base is added, the solution turns basic.
The total initial amount of the hydronium ions is:
[latex]\text{n(H}^{+})_0 = [\text{H}_3\text{O}^{+}]_0\;\times\;0.02500\;\text{L} = 0.002500\;\text{mol}[/latex]
One time X mL of the 0.100-Thousand base solution is added, the number of moles of the OH− ions introduced is:
[latex]\text{north(OH}^{-})_0 = 0.100\;Grand\;\times\;\text{X\;mL}\;\times\;(\frac{1\;\text{L}}{1000\;\text{mL}})[/latex]
The total volume becomes: [latex]5 = (25.00\;\text{mL}\;+\;\text{X\;mL})(\frac{ane\;\text{50}}{g\;\text{mL}})[/latex]
The number of moles of H3O+ becomes:
[latex]\text{due north(H}^{+}) = \text{n(H}^{+})_0\;-\;\text{north(OH}^{-})_0 = 0.002500\;\text{mol}\;-\;0.100\;Yard\;\times\;\text{X\;mL}\;\times\;(\frac{i\;\text{L}}{thou\;\text{mL}})[/latex]
The concentration of H3O+ is:
[latex][\text{H}_3\text{O}^{+}] = \frac{\text{n(H}^{+})}{5} = \frac{0.002500\;\text{mol}\;-\;0.100\;1000\;\times\;\text{X\;mL}\;\times\;(\frac{1\;\text{Fifty}}{m\;\text{mL}})}{(25.00\;\text{mL}\;+\;\text{Ten\;mL})(\frac{i\;\text{L}}{1000\;\text{mL}})}[/latex]
[latex]= \frac{0.002500\;\text{mol}\;\times\;(\frac{1000\;\text{mL}}{1\;\text{50}})\;-\;0.100\;Grand\;\times\;\text{10\;mL}}{25.00\;\text{mL}\;+\;\text{Ten\;mL}}[/latex]
[latex]\text{pH} = -\text{log}([\text{H}_3\text{O}^{+}])[/latex]
The preceding calculations work if [latex]\text{due north(H}^{+})_0\;-\;\text{n(OH}^{-})_0\;{\textgreater}\;0[/latex] and and then n(H+) > 0. When [latex]\text{n(H}^{+})_0 = \text{n(OH}^{-})_0[/latex], the HthreeO+ ions from the acid and the OH− ions from the base mutually neutralize. At this point, the only hydronium ions left are those from the autoionization of water, and there are no OH− particles to neutralize them. Therefore, in this case:
[latex][\text{H}_3\text{O}^{+}] = [\text{OH}^{-}]\text{,\;}[\text{H}_3\text{O}^{+}] = K_{\text{w}} = ane.0\;\times\;10^{-xiv}\text{;\;}[\text{H}_3\text{O}^{+}] = 1.0\;\times\;10^{-7}[/latex]
[latex]\text{pH} = -\text{log}(1.0\;\times\;ten^{-7}) = 7.00[/latex]
Finally, when [latex]\text{n(OH}^{-})_0\;{\textgreater}\;\text{northward(H}^{+})_0[/latex], there are non enough H3O+ ions to neutralize all the OH− ions, and instead of [latex]\text{due north(H}^{+}) = \text{north(H}^{+})_0\;-\;\text{n(OH}^{-})_0[/latex], we calculate: [latex]\text{n(OH}^{-}) = \text{northward(OH}^{-})_0\;-\;\text{n(H}^{+})_0[/latex]
In this case:
[latex][\text{OH}^{-}] = \frac{\text{n(OH}^{-})}{5} = \frac{0.100\;M\;\times\;\text{X\;mL}\;\times\;(\frac{1\;\text{50}}{1000\;\text{mL}})\;-\;0.002500\;\text{mol}}{(25.00\;\text{mL}\;+\;\text{X\;mL})(\frac{1\;\text{L}}{k\;\text{mL}})}[/latex]
[latex]= \frac{0.100\;1000\;\times\;\text{X\;mL}\;-\;0.002500\;\text{mol}\;\times\;(\frac{1000\;\text{mL}}{i\;\text{Fifty}})}{25.00\;\text{mL}\;+\;\text{Ten\;mL}}[/latex]
[latex]\text{pH} = 14\;-\;\text{pOH} = 14\;+\;\text{log}([\text{OH}^{-}])[/latex]
Let united states now consider the four specific cases presented in this problem:
(a) X = 0 mL
[latex][\text{H}_3\text{O}^{+}] = \frac{\text{n(H}^{+})}{V} = \frac{0.002500\;\text{mol}\;\times\;(\frac{1000\;\text{mL}}{1\;\text{50}})}{25.00\;\text{mL}} = 0.1\;M[/latex]
pH = −log(0.100) = i.000
(b) X = 12.50 mL
[latex][\text{H}_3\text{O}^{+}] = \frac{\text{northward(H}^{+})}{V} = \frac{0.002500\;\text{mol}\;\times\;(\frac{1000\;\text{mL}}{1\;\text{L}})\;-\;0.100\;Thou\;\times\;12.50\;\text{mL}}{25.00\;\text{mL}\;+\;12.50\;\text{mL}} = 0.0333\;M[/latex]
pH = −log(0.0333) = 1.477
(c) 10 = 25.00 mL
Since the volumes and concentrations of the acid and base solutions are the aforementioned: [latex]\text{n(H}^{+})_0 = \text{n(OH}^{-})_0[/latex], and pH = 7.000, as described earlier.
(d) X = 37.fifty mL
In this example:
[latex]\text{n(OH}^{-})_0\;{\textgreater}\;\text{n(H}^{+})_0[/latex]
[latex][\text{OH}^{-}] = \frac{\text{n(OH}^{-})}{Five} = \frac{0.100\;M\;\times\;35.70\;\text{mL}\;-\;0.002500\;\text{mol}\;\times\;(\frac{one thousand\;\text{mL}}{one\;\text{L}})}{25.00\;\text{mL}\;+\;37.50\;\text{mL}} = 0.0200\;1000[/latex]
pH = 14 − pOH = 14 + log([OH−]) = xiv + log(0.0200) = 12.30
Check Your Learning
Calculate the pH for the strong acid/strong base titration between 50.0 mL of 0.100 M HNOthree(aq) and 0.200 One thousand NaOH (titrant) at the listed volumes of added base: 0.00 mL, 15.0 mL, 25.0 mL, and 40.0 mL.
Reply:
0.00: 1.000; 15.0: 1.5111; 25.0: 7; xl.0: 12.523
In the example, we calculated pH at four points during a titration. Tabular array 4 shows a detailed sequence of changes in the pH of a stiff acrid and a weak acid in a titration with NaOH.
| Volume of 0.100 M NaOH Added (mL) | Moles of NaOH Added | pH Values 0.100 Grand HCl[ane] | pH Values 0.100 Thousand CH3CO2H[2] |
|---|---|---|---|
| 0.0 | 0.0 | 1.00 | 2.87 |
| 5.0 | 0.00050 | 1.xviii | 4.14 |
| 10.0 | 0.00100 | one.37 | 4.57 |
| xv.0 | 0.00150 | 1.60 | iv.92 |
| 20.0 | 0.00200 | 1.95 | five.35 |
| 22.0 | 0.00220 | two.twenty | 5.61 |
| 24.0 | 0.00240 | ii.69 | half dozen.13 |
| 24.5 | 0.00245 | 3.00 | 6.44 |
| 24.nine | 0.00249 | 3.70 | seven.14 |
| 25.0 | 0.00250 | 7.00 | 8.72 |
| 25.ane | 0.00251 | 10.30 | 10.30 |
| 25.5 | 0.00255 | eleven.00 | xi.00 |
| 26.0 | 0.00260 | eleven.29 | 11.29 |
| 28.0 | 0.00280 | 11.75 | xi.75 |
| 30.0 | 0.00300 | xi.96 | 11.96 |
| 35.0 | 0.00350 | 12.22 | 12.22 |
| twoscore.0 | 0.00400 | 12.36 | 12.36 |
| 45.0 | 0.00450 | 12.46 | 12.46 |
| fifty.0 | 0.00500 | 12.52 | 12.52 |
| Table 4. pH Values in the Titrations of a Strong Acrid with a Strong Base and of a Weak Acid with a Strong Base | |||
The simplest acid-base reactions are those of a potent acid with a strong base. Tabular array 4 shows information for the titration of a 25.0-mL sample of 0.100 M hydrochloric acrid with 0.100 M sodium hydroxide. The values of the pH measured after successive additions of small amounts of NaOH are listed in the outset cavalcade of this table, and are graphed in Figure 1, in a form that is called a titration bend. The pH increases slowly at get-go, increases rapidly in the middle portion of the curve, and so increases slowly again. The bespeak of inflection (located at the midpoint of the vertical office of the curve) is the equivalence point for the titration. It indicates when equivalent quantities of acid and base are present. For the titration of a stiff acid with a strong base of operations, the equivalence bespeak occurs at a pH of 7.00 and the points on the titration bend can exist calculated using solution stoichiometry (Table 4 and Effigy 1).
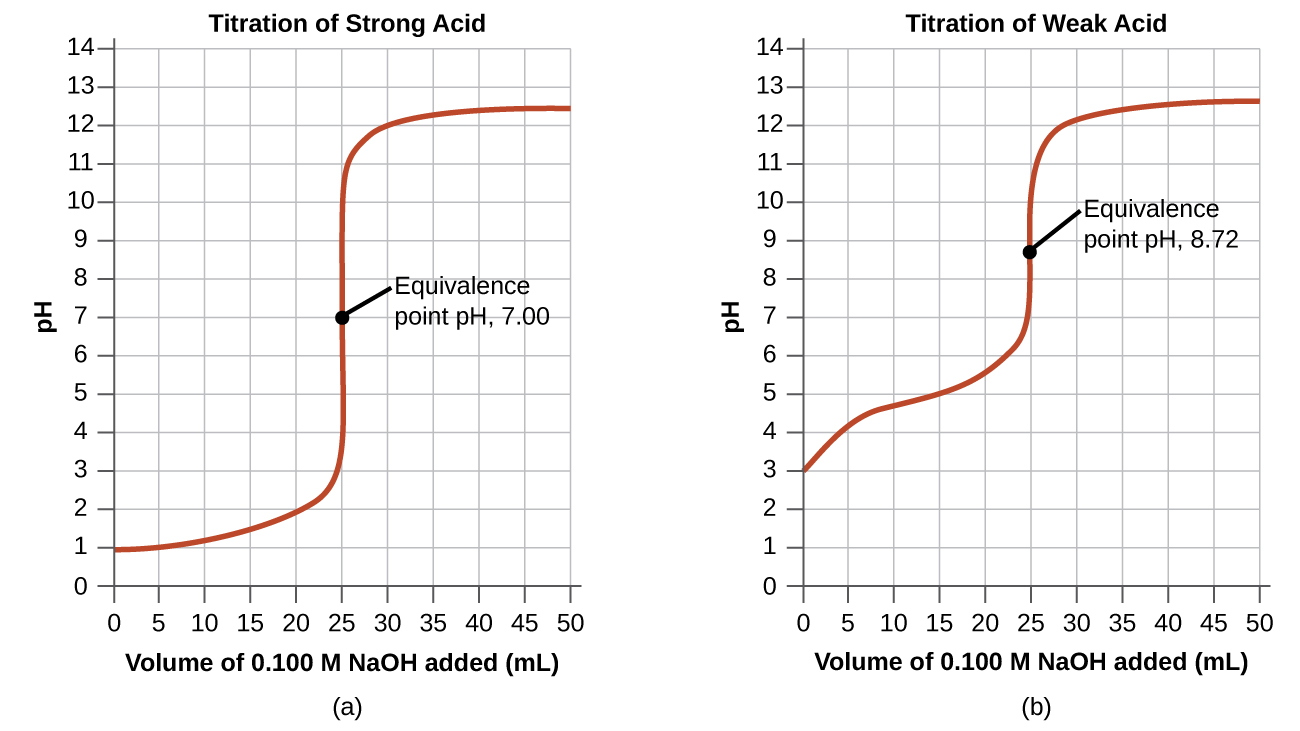
The titration of a weak acid with a strong base of operations (or of a weak base with a strong acid) is somewhat more than complicated than that just discussed, merely it follows the same general principles. Let us consider the titration of 25.0 mL of 0.100 1000 acetic acid (a weak acid) with 0.100 G sodium hydroxide and compare the titration curve with that of the potent acid. Table iv gives the pH values during the titration, Effigy ane shows the titration curve.
Although the initial volume and molarity of the acids are the same, there are important differences between the two titration curves. The titration curve for the weak acrid begins at a higher value (less acidic) and maintains higher pH values upward to the equivalence signal. This is because acetic acid is a weak acid, which is simply partially ionized. The pH at the equivalence signal is also college (8.72 rather than 7.00) due to the hydrolysis of acetate, a weak base of operations that raises the pH:
[latex]\text{CH}_3\text{CO}_2^{\;\;-}(aq)\;+\;\text{H}_2\text{O}(fifty)\;{\leftrightharpoons}\;\text{CH}_3\text{CO}_2\text{H}(l)\;+\;\text{OH}^{-}(aq)[/latex]
After the equivalence bespeak, the 2 curves are identical because the pH is dependent on the excess of hydroxide ion in both cases.
Example 2
Titration of a Weak Acid with a Strong Base
The titration curve shown in Figure iii is for the titration of 25.00 mL of 0.100 M CHiiiCO2H with 0.100 K NaOH. The reaction tin can be represented as:
[latex]\text{CH}_3\text{CO}_2\text{H}\;+\;\text{OH}^{-}\;{\longrightarrow}\;\text{CH}_3\text{CO}_2^{\;\;-}\;+\;\text{H}_2\text{O}[/latex]
(a) What is the initial pH before any amount of the NaOH solution has been added? K a = 1.eight × ten−5 for CHthreeCOtwoH.
(b) Notice the pH after 25.00 mL of the NaOH solution have been added.
(c) Find the pH after 12.50 mL of the NaOH solution has been added.
(d) Observe the pH after 37.50 mL of the NaOH solution has been added.
Solution
(a) Assuming that the dissociated amount is pocket-size compared to 0.100 M, we observe that:
[latex]K_{\text{a}} = \frac{[\text{H}_3\text{O}^{+}][\text{CH}_3\text{CO}_2^{\;\;-}]}{[\text{CH}_3\text{CO}_2\text{H}]}\;{\approx}\;\frac{[\text{H}_3\text{O}^{+}]^two}{[\text{CH}_3\text{CO}_2\text{H}]_0}[/latex], and [latex][\text{H}_3\text{O}^{+}] = \sqrt{K_{\text{a}}\;\times\;[\text{CH}_3\text{CO}_2\text{H}]} = \sqrt{ane.8\;\times\;10^{-5}\;\times\;0.100} = 1.three\;\times\;10^{-3}[/latex]
[latex]\text{pH} = -\text{log}(1.three\;\times\;x^{-iii}) = 2.87[/latex]
(b) After 25.00 mL of NaOH are added, the number of moles of NaOH and CH3CO2H are equal considering the amounts of the solutions and their concentrations are the same. All of the CH3CO2H has been converted to [latex]\text{CH}_3\text{CO}_2^{\;\;-}[/latex]. The concentration of the [latex]\text{CH}_3\text{CO}_2^{\;\;-}[/latex] ion is:
[latex]\frac{0.00250\;\text{mol}}{0.0500\;\text{L}} = 0.0500\;M\;\text{CH}_3\text{CO}_2^{\;\;-}[/latex]
The equilibrium that must be focused on now is the basicity equilibrium for [latex]\text{CH}_3\text{CO}_2^{\;\;-}[/latex]:
[latex]\text{CH}_3\text{CO}_2^{\;\;-}(aq)\;+\;\text{H}_2\text{O}(50)\;{\rightleftharpoons}\;\text{CH}_3\text{CO}_2\text{H}(aq)\;+\;\text{OH}^{-}(aq)[/latex]
and so we must decide One thousand b for the base of operations by using the ion product abiding for water:
[latex]K_{\text{b}} = \frac{[\text{CH}_3\text{CO}_2\text{H}][\text{OH}^{-}]}{[\text{CH}_3\text{CO}_2^{\;\;-}]}[/latex]
[latex]K_{\text{a}} = \frac{[\text{CH}_3\text{CO}_2^{\;\;-}][\text{H}^{+}]}{[\text{CH}_3\text{CO}_2\text{H}]}\text{,\;and so\;}\frac{[\text{CH}_3\text{CO}_2\text{H}]}{[\text{CH}_3\text{CO}_2^{\;\;-}]} = \frac{[\text{H}^{+}]}{K_{\text{a}}}[/latex].
Since Chiliad w = [H+][OH−]:
[latex]K_{\text{b}} = \frac{[\text{H}^{+}][\text{OH}^{-}]}{K_{\text{a}}} = \frac{K_{\text{westward}}}{K_{\text{a}}} = \frac{one.0\;\times\;x^{-14}}{1.viii\;\times\;10^{-5}} = 5.6\;\times\;10^{-10}[/latex]
Permit us denote the concentration of each of the products of this reaction, CH3CO2H and OH−, as x. Using the assumption that x is minor compared to 0.0500 Thousand, [latex]K_{\text{b}} = \frac{x^{2}}{0.0500\;Thousand}[/latex], and and so:
[latex]x = [\text{OH}^{-}] = 5.3\;\times\;x^{-six}[/latex]
[latex]\text{pOH} = -\text{log}(5.three\;\times\;ten^{-6}) = v.28[/latex]
[latex]\text{pH} = 14.00\;-\;five.28 = 8.72[/latex]
Note that the pH at the equivalence indicate of this titration is significantly greater than 7.
(c) In (a), 25.00 mL of the NaOH solution was added, and then practically all the CHthreeCO2H was converted into [latex]\text{CH}_3\text{CO}_2^{\;\;-}[/latex]. In this case, only 12.50 mL of the base solution has been introduced, and then only half of all the CH3COiiH is converted into [latex]\text{CH}_3\text{CO}_2^{\;\;-}[/latex]. The total initial number of moles of CHthreeCO2H is 0.02500L × 0.100 Thou = 0.00250 mol, so afterward adding the NaOH, the numbers of moles of CH3CO2H and [latex]\text{CH}_3\text{CO}_2^{\;\;-}[/latex] are both approximately equal to [latex]\frac{0.00250\;\text{mol}}{two} = 0.00125\;\text{mol}[/latex], and their concentrations are the same.
Since the amount of the added base is smaller than the original amount of the acid, the equivalence point has not been reached, the solution remains a buffer, and nosotros tin use the Henderson-Hasselbalch equation:
[latex]\text{pH} = \text{p}K_{\text{a}}\;+\;\text{log}\frac{[\text{Base}]}{[\text{Acid}]} = -\text{log}(K_{\text{a}})\;+\;\text{log}\frac{[\text{CH}_3\text{CO}_2^{\;\;-}]}{[\text{CH}_3\text{CO}_2\text{H}]} = -\text{log}(one.8\;\times\;10^{-5})\;+\;\text{log}(1)[/latex]
(as the concentrations of [latex]\text{CH}_3\text{CO}_2^{\;\;-}[/latex] and CH3COtwoH are the aforementioned)
Thus:
[latex]\text{pH} = -\text{log}(ane.viii\;\times\;10^{-5}) = 4.74[/latex]
(the pH = the pK a at the halfway indicate in a titration of a weak acid)
(d) Later on 37.50 mL of NaOH is added, the amount of NaOH is 0.03750 L × 0.100 One thousand = 0.003750 mol NaOH. Since this is by the equivalence point, the excess hydroxide ions will make the solution bones, and nosotros can once again use stoichiometric calculations to make up one's mind the pH:
[latex][\text{OH}^{-}] = \frac{(0.003750\;\text{mol}\;-\;0.00250\;\text{mol})}{0.06250\;\text{50}} = ii.00\;\times\;x^{-2}\;K[/latex]
Then:
[latex]\text{pOH} = -\text{log}(ii.00\;\times\;10^{-2}) = 1.lxx\text{,\;and\;pH} = 14.00\;-\;i.70 = 12.30[/latex]
Note that this upshot is the same as for the potent acid-strong base titration instance provided, since the amount of the strong base added moves the solution past the equivalence point.
Check Your Learning
Calculate the pH for the weak acid/strong base titration betwixt fifty.0 mL of 0.100 Chiliad HCOOH(aq) (formic acid) and 0.200 M NaOH (titrant) at the listed volumes of added base: 0.00 mL, 15.0 mL, 25.0 mL, and 30.0 mL.
Reply:
0.00 mL: 2.37; 15.0 mL: iii.92; 25.00 mL: viii.29; 30.0 mL: 12.097
Acid-Base Indicators
Sure organic substances modify color in dilute solution when the hydronium ion concentration reaches a detail value. For example, phenolphthalein is a colorless substance in whatsoever aqueous solution with a hydronium ion concentration greater than 5.0 × 10−nine One thousand (pH < 8.3). In more basic solutions where the hydronium ion concentration is less than 5.0 × 10−nine M (pH > 8.iii), it is ruddy or pink. Substances such as phenolphthalein, which can be used to make up one's mind the pH of a solution, are chosen acid-base of operations indicators. Acid-base of operations indicators are either weak organic acids or weak organic bases.
The equilibrium in a solution of the acid-base indicator methyl orange, a weak acid, tin can exist represented past an equation in which we use HIn as a simple representation for the complex methyl orange molecule:
[latex]\begin{array}{ccccccc} \text{HIn}(aq) & + & \text{H}_2\text{O}(l) & {\leftrightharpoons} & \text{H}_3\text{O}^{+}(aq) & + & \text{In}^{-}(aq) \\[0.5em] \text{ruby-red} & & & & & & \text{yellow} \end{assortment}[/latex]
[latex]K_{\text{a}} = \frac{[\text{H}_3\text{O}^{+}][\text{In}^{-}]}{[\text{HIn}]} = four.0\;\times\;10^{-4}[/latex]
The anion of methyl orangish, In−, is yellow, and the nonionized course, HIn, is ruby-red. When we add acid to a solution of methyl orange, the increased hydronium ion concentration shifts the equilibrium toward the nonionized red form, in accordance with Le Châtelier's principle. If we add base, we shift the equilibrium towards the yellowish course. This behavior is completely analogous to the activeness of buffers.
An indicator's color is the visible issue of the ratio of the concentrations of the two species In− and HIn. If most of the indicator (typically about threescore−90% or more than) is present every bit In−, and then we see the color of the In− ion, which would be yellow for methyl orange. If most is present as HIn, then we see the color of the HIn molecule: red for methyl orange. For methyl orange, we tin can rearrange the equation for Grand a and write:
[latex]\frac{[\text{In}^{-}]}{[\text{HIn}]} = \frac{[\text{substance\;with\;yellow\;color}]}{[\text{substance\;with\;red\;color}]} = \frac{K_{\text{a}}}{[\text{H}_3\text{O}^{+}]}[/latex]
This shows us how the ratio of [latex]\frac{[\text{In}^{-}]}{[\text{HIn}]}[/latex] varies with the concentration of hydronium ion.
The in a higher place expression describing the indicator equilibrium tin be rearranged:
[latex]\frac{[\text{H}_3\text{O}^{+}]}{K_{\text{a}}} = \frac{[\text{HIn}]}{[\text{In}^{-}]}[/latex]
[latex]\text{log}(\frac{[\text{H}_3\text{O}^{+}]}{K_{\text{a}}}) = \text{log}(\frac{[\text{HIn}]}{[\text{In}^{-}]})[/latex]
[latex]\text{log}([\text{H}_3\text{O}^{+}])\;-\;\text{log}(K_{\text{a}}) = -\text{log}(\frac{[\text{In}^{-}]}{[\text{HIn}]})[/latex]
[latex]-\text{pH}\;+\;\text{p}K_{\text{a}} = -\text{log}(\frac{[\text{In}^{-}]}{[\text{HIn}]})[/latex]
[latex]\text{pH} = \text{p}K_{\text{a}}\;+\;\text{log}(\frac{[\text{In}^{-}]}{[\text{HIn}]})\;\text{or\;pH} = \text{p}K_{\text{a}}\;+\;\text{log}(\frac{[\text{base of operations}]}{[\text{acid}]})[/latex]
The last formula is the aforementioned every bit the Henderson-Hasselbalch equation, which can be used to depict the equilibrium of indicators.
When [HthreeO+] has the same numerical value as K a, the ratio of [In−] to [HIn] is equal to 1, meaning that 50% of the indicator is present in the cherry form (HIn) and fifty% is in the yellow ionic class (In−), and the solution appears orange in color. When the hydronium ion concentration increases to 8 × 10−four M (a pH of 3.1), the solution turns red. No change in color is visible for whatsoever further increase in the hydronium ion concentration (subtract in pH). At a hydronium ion concentration of 4 × 10−v Chiliad (a pH of 4.four), well-nigh of the indicator is in the yellowish ionic form, and a further decrease in the hydronium ion concentration (increase in pH) does not produce a visible color change. The pH range between 3.i (blood-red) and iv.4 (yellow) is the color-change interval of methyl orange; the pronounced color change takes place betwixt these pH values.
There are many different acrid-base indicators that encompass a wide range of pH values and can be used to make up one's mind the guess pH of an unknown solution by a process of elimination. Universal indicators and pH newspaper incorporate a mixture of indicators and exhibit different colors at different pHs. Figure 2 presents several indicators, their colors, and their color-change intervals.
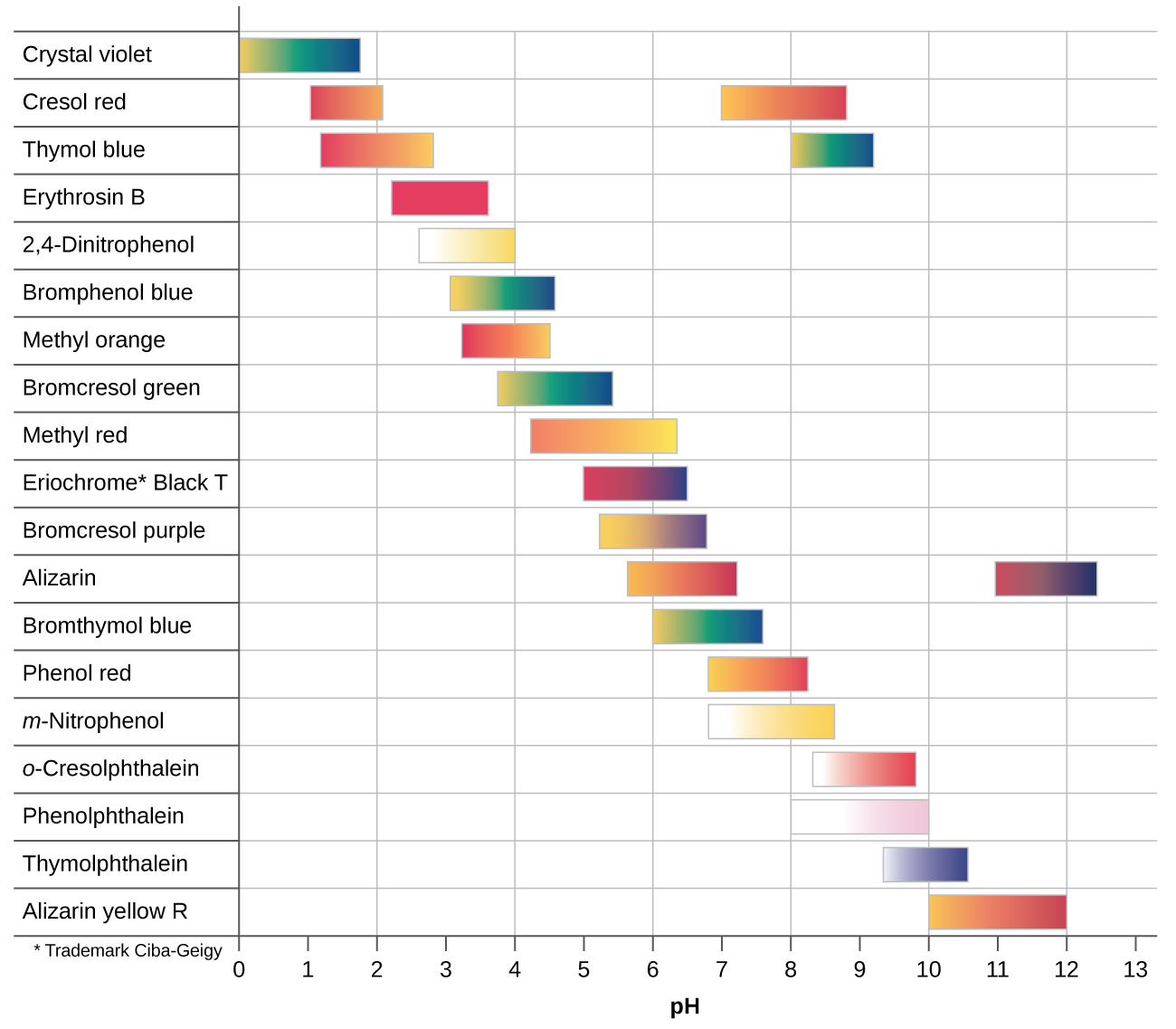
Titration curves help the states pick an indicator that volition provide a sharp color modify at the equivalence signal. The best selection would be an indicator that has a color alter interval that brackets the pH at the equivalence signal of the titration.
The color change intervals of three indicators are shown in Effigy 3. The equivalence points of both the titration of the strong acid and of the weak acrid are located in the color-change interval of phenolphthalein. Nosotros can use it for titrations of either strong acid with strong base or weak acrid with stiff base.
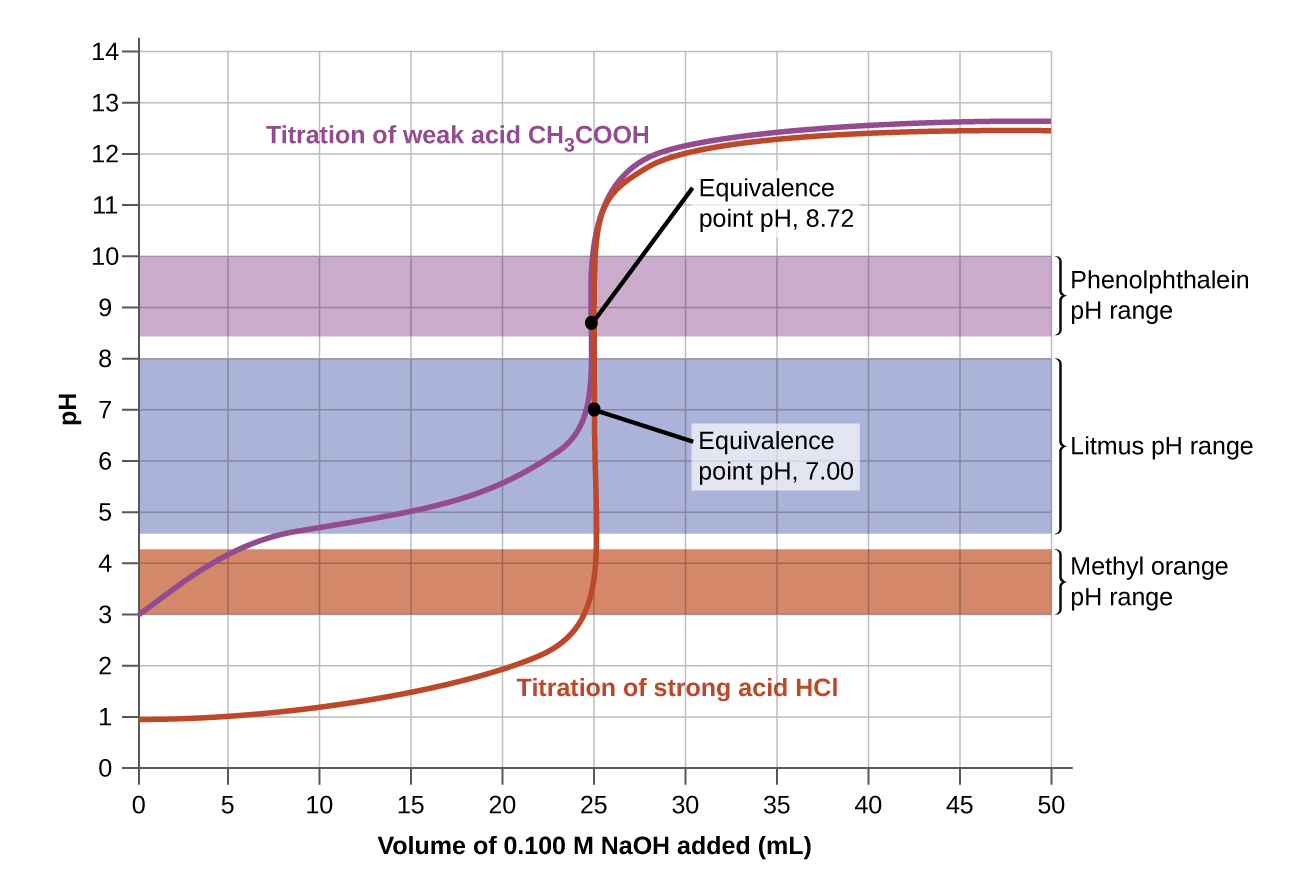
Litmus is a suitable indicator for the HCl titration because its colour change brackets the equivalence point. However, we should non use litmus for the CH3CO2H titration because the pH is within the color-change interval of litmus when simply nigh 12 mL of NaOH has been added, and it does non exit the range until 25 mL has been added. The color modify would be very gradual, taking place during the addition of 13 mL of NaOH, making litmus useless as an indicator of the equivalence point.
We could use methyl orangish for the HCl titration, but it would non give very accurate results: (1) It completes its color change slightly before the equivalence signal is reached (only very close to it, so this is non too serious); (2) it changes colour, every bit Effigy three shows, during the addition of near 0.5 mL of NaOH, which is not so sharp a color change every bit that of litmus or phenolphthalein; and (3) it goes from yellow to orangish to red, making detection of a precise endpoint much more challenging than the colorless to pinkish modify of phenolphthalein. Effigy three shows us that methyl orange would exist completely useless every bit an indicator for the CH3CO2H titration. Its color change begins afterwards most 1 mL of NaOH has been added and ends when well-nigh 8 mL has been added. The colour change is completed long earlier the equivalence point (which occurs when 25.0 mL of NaOH has been added) is reached and hence provides no indication of the equivalence point.
We base of operations our choice of indicator on a calculated pH, the pH at the equivalence bespeak. At the equivalence point, equimolar amounts of acrid and base of operations have been mixed, and the calculation becomes that of the pH of a solution of the salt resulting from the titration.
Primal Concepts and Summary
A titration curve is a graph that relates the change in pH of an acidic or basic solution to the book of added titrant. The characteristics of the titration curve are dependent on the specific solutions being titrated. The pH of the solution at the equivalence bespeak may be greater than, equal to, or less than 7.00. The pick of an indicator for a given titration depends on the expected pH at the equivalence point of the titration, and the range of the colour modify of the indicator.
Chemistry End of Chapter Exercises
- Explain how to cull the advisable acid-base indicator for the titration of a weak base with a stiff acrid.
- Explain why an acid-base indicator changes color over a range of pH values rather than at a specific pH.
- Why can we ignore the contribution of water to the concentrations of H3O+ in the solutions of following acids:
0.0092 M HClO, a weak acid
0.0810 Yard HCN, a weak acid
0.120 M [latex]\text{Fe(H}_2\text{O})_6^{\;\;two+}[/latex] a weak acrid, K a = 1.half dozen × 10−vii
only not the contribution of water to the concentration of OH−?
- We can ignore the contribution of water to the concentration of OH− in a solution of the following bases:
0.0784 M C6H5NH2, a weak base
0.11 K (CH3)3Due north, a weak base
but not the contribution of water to the concentration of HiiiO+?
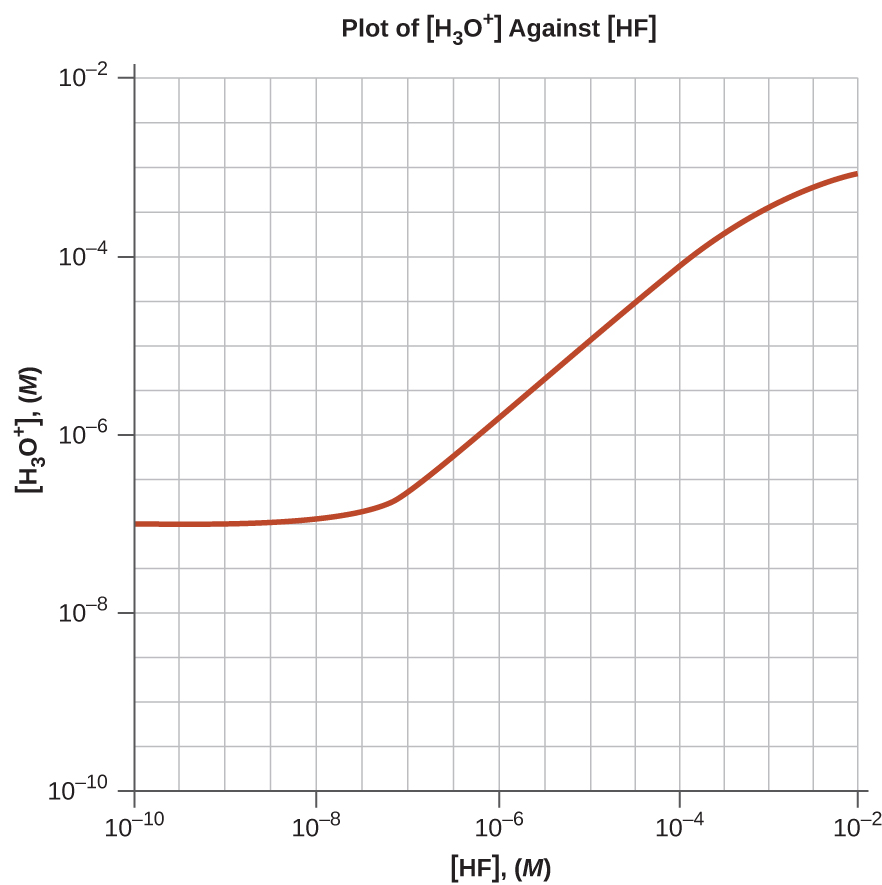
- Draw a curve for a series of solutions of HF. Plot [H3O+]total on the vertical axis and the total concentration of HF (the sum of the concentrations of both the ionized and nonionized HF molecules) on the horizontal axis. Let the total concentration of HF vary from 1 × x−10 M to ane × 10−2 Chiliad.
- Depict a bend similar to that shown in Effigy iii for a series of solutions of NH3. Plot [OH−] on the vertical axis and the total concentration of NH3 (both ionized and nonionized NH3 molecules) on the horizontal axis. Let the total concentration of NHiii vary from 1 × x−10 M to ane × 10−2 M.
- Calculate the pH at the following points in a titration of 40 mL (0.040 L) of 0.100 M barbituric acid (K a = nine.eight × 10−v) with 0.100 M KOH.
(a) no KOH added
(b) xx mL of KOH solution added
(c) 39 mL of KOH solution added
(d) 40 mL of KOH solution added
(due east) 41 mL of KOH solution added
- The indicator dinitrophenol is an acrid with a Yard a of 1.1 × 10−4. In a ane.0 × 10−four–Yard solution, it is colorless in acrid and yellow in base of operations. Calculate the pH range over which it goes from ten% ionized (colorless) to 90% ionized (yellow).
Glossary
- acid-base of operations indicator
- organic acid or base whose colour changes depending on the pH of the solution information technology is in
- colour-change interval
- range in pH over which the color change of an indicator takes place
- titration curve
- plot of the pH of a solution of acid or base of operations versus the volume of base or acid added during a titration
Solutions
Answers to Chemistry End of Affiliate Exercises
i. At the equivalence signal in the titration of a weak base with a strong acrid, the resulting solution is slightly acidic due to the presence of the cohabit acid. Thus, pick an indicator that changes colour in the acidic range and brackets the pH at the equivalence bespeak. Methyl orangish is a practiced example.
3. In an acrid solution, the simply source of OH− ions is h2o. We use K west to calculate the concentration. If the contribution from water was neglected, the concentration of OH− would be zero.
v.
vii. (a) pH = 2.50;
(b) pH = 4.01;
(c) pH = five.60;
(d) pH = 8.35;
(eastward) pH = eleven.08
Source: https://opentextbc.ca/chemistry/chapter/14-7-acid-base-titrations/
0 Response to "if 50 ml of .114 m hno3 was added to 40 ml of the previous solution, what is the ph"
Post a Comment